EDMUS 5.7.1
EDMUS 5.7 has been available since March 11, 2019, and EDMUS 5.7.1 since May 6, 2019.
In EDMUS 5.7, the following new or improved features have been implemented:
-
- New "Vital Status" fields
- Instead of the simple "Deceased" checkbox of previous versions, it is now possible to indicate whether the patient is alive or deceased.
By default, the "Alive" date is the date of last information of the EDMUS file, but the user can specify a later date.
In addition, the decease date can be marked as "Unknown" (and a date of information can then be specified).



-
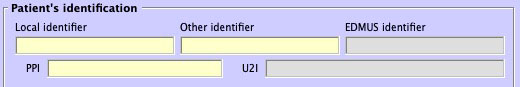
- New "PPI" field
- For the "Permanent Patient Identifier", a permament patient identifier code in a given hospital.
-
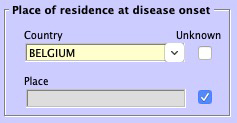
- Unknown place of residence at disease onset
- The place of residence at disease onset can now be marked as "Unknown".
-
- Clinical form: NMORD
- The notion of "NMORD" (NMO related disorder) has been added.

-
- Improved calculation of the "Last clinical follow-up" and "Last information" dates
- The "Last clinical follow-up" and "Last information" dates now ignore the decease date.
The "Last information" date now ignores the information date for stopped disease modifying treatments marked as "Unknown dates"; it now takes into account the dates of birth of the patient's children.
The date of the last clinical assessment is now displayed.

-
- Unknown dates
- It is now possible to specify that a given level of irreversible motor disability has been reached at an "Unknown Date".

-
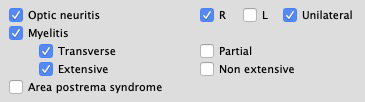
- Myelitis
- Addition of a "Myelitis" checkbox, with "Transverse"/"Partial" and "Extensive"/"Non extensive" checkboxes.
-
- Syndromes
- "Optic neuritis", "Myelitis" and "Area postrema syndrome" (replacing "Intractable hiccup/Nausea") have been grouped at the end of the semiology tab.
-
- Progression
- A new question has been added, to indicate whether the disease has progressed (according to Lublin 2014 criteria) in the 12 months preceding this Clinical Assessment. This is freely specified by the neurologist, without control or link with other data.

-
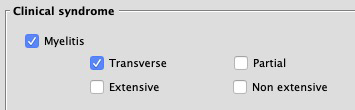
- Myelitis, Area postrema syndrome
- As in the Neurological Episodes panel, a "Myelitis" checkbox has been added, with "Transverse"/"Partial" and "Extensive"/"Non extensive" checkboxes. And "Intractable hiccup/Nausea" has been replaced by "Area postrema syndrome".

-
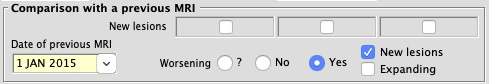
- Improved comparison with a previous MRI
- The "Unchanged"/"Worsened"/"Improved" checkboxes have been replaced by the notion of Worsening (Yes or No); if "Yes",
checkboxes for New or Expanding lesions become available.
This relates to the global comparison with a previous MRI. The more precise "New" checkboxes for "T1", "T1/Gado" and "T2/PD" remain available.
-
- Improved assessment of oligoclonal bands
- When oligoclonal bands are present, a distinction is now made between "Yes: 1 band" and "Yes: >= 2 bands". Only the presence of >= 2 bands is taken
into account in the "inflammatory CSF" criterion of the Diagnosis panel.
When retrieving EDMUS 5.5 data, CSF exams for which "Yes" was selected are recoded as "Yes: >= 2 bands", unless the number of bands was specified as 1.

-
- Link to a treatment
- To link an Adverse Event (AE) to a treatment, it is now requested to specify that a causal relationship is suspected with the treatment.
(In previous versions, it was possible to link an AE to a treatment, while at the same time specifying "Treatment relationship suspected" as "No" or "?". When retrieving EDMUS 5.5 data, AE where this question was marked as "?" will be marked as "Yes"; see "Data Checkup" below).

-
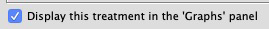
- Display in Graphs
- It is now possible to specify that a given treatment is to be displayed in the "Graphs" panel.
-
- Display of Samples
- Records in the "Samples" panel are now displayed in the "Graphs" panel.

-
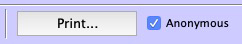
- "Anonymous" printing
- Graphs can now be printed in an "Anonymous" manner, i.e. without displaying the name and ID of the patient.
-
- New tests
- New tests have been added:
- Missing data: place of residence at disease onset
- Missing data: date of death
- Inconsistent data: date of death
(e.g. adverse event with a date of death different from the date mentioned in the Personal Data panel) - Data to check: disease with no seriousness specified
- Data to check: adverse event with no seriousness specified
- Data to check: disease-modifying treatment with an INN not listed in the EDMUS thesaurus
- Data to check: relapse therapy with an INN not listed in the EDMUS thesaurus
- Data to check: CSF exam with oligoclonal banding recorded before EDMUS 5.7
(to identify CSF records recoded because of the new specification of the number of bands) - Data to check: adverse event with treatment causal relationship recorded before EDMUS 5.7
(to identify AE linked to a treatment but where "Treatment relationship suspected" was marked as "?") - Data to check: disease-modifying treatment with inconsistent dates and status
(e.g. ongoing date later than the end date)
-
- Improved form
- The OFSEP form has been improved, and modified to take the new features into account.

-
- Updated export
- The export files have been modified to take the new features into account.
In addition, a lot of small improvements and bug fixes have been implemented.
EDMUS 5.7.1 fixes two issues in the Graphs panel: when printing, the word "Death" was erroneously added; and EDMUS was quitting when trying to print graphs for patients without any disease-modifying treatment nor relapse therapy.